Iimbonakalo
I-electrode ye-ion ekwi-intanethi ilinganiswa kwisisombululo samanzi se-chlorine ion concentration okanye ukumisela umda kunye ne-electrode yesalathisi i-fluorine/chlorine ions ukwenza ii-complexes ezizinzileyo ze-ion concentration.
| Umgaqo wokulinganisa | I-ion selective potentiometry |
| Uluhlu lokulinganisa | 0.0~2300mg/L |
| Ubushushu obuzenzekelayouluhlu lwembuyekezo | 0~99.9℃,nge-25℃ njengeubushushu obubhekisele kuyo |
| Uluhlu lobushushu | 0~99.9℃ |
| Ubushushu obuzenzekelayoimbuyekezo | 2.252K,10K,PT100,PT1000etc |
| Isampuli yamanzi ivavanyiwe | 0~99.9℃,0.6MPa |
| Ii-ion zokuphazamisa | AL3+,Fe3+,OH-njl. njl. |
| uluhlu lwexabiso le-pH | 5.00~10.00PH |
| Amandla angenanto | > 200mV (amanzi acocekileyo) |
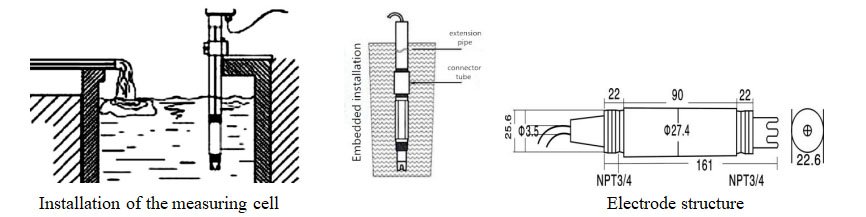
| Ubude be-electrode | 195mm |
| Izinto ezisisiseko | I-PPS |
| Umsonto we-electrode | Intambo yombhobho eyi-3/4(IsiNgesi)I-NPT) |
| Ubude bekhebula | Iimitha ezi-5 |
I-ion yi-atom okanye i-molecule etshajiweyo. Itshajiswa kuba inani lee-electron alilingani nenani lee-proton kwi-atom okanye kwi-molecule. I-atom inokufumana i-charge entle okanye i-charge embi kuxhomekeke ekubeni inani lee-electron kwi-atom likhulu okanye lingaphantsi kunenani lee-proton kwi-atom.
Xa iathom itsalelwa kwenye iathom kuba inenani elingalinganiyo lee-electron kunye neeproton, iathom ibizwa ngokuba yi-ION. Ukuba iathom inee-electron ezininzi kunee-proton, yi-ion engalunganga, okanye i-ANION. Ukuba inee-proton ezininzi kunee-electron, yi-ion engalunganga.















