Uthotho lwee-electrode zemizi-mveliso ezisebenzisa i-conductivity zisetyenziswa ngokukodwa ekulinganiseni ixabiso le-conductivity lamanzi acocekileyo, amanzi acocekileyo kakhulu, unyango lwamanzi, njl. Ifanelekile ngokukodwa ekulinganiseni i-conductivity kwisikhululo samandla obushushu nakwishishini lokunyanga amanzi. Ibonakala ngesakhiwo se-double-cylinder kunye nezinto ze-titanium alloy, ezinoku-oxidized ngokwemvelo ukuze zenze i-chemical passivation. Umphezulu wayo othintela ukungena kwamanzi umelana nazo zonke iintlobo zolwelo ngaphandle kwe-fluoride acid. Izinto ezihlawulela ubushushu zezi: NTC2.252K, 2K, 10K, 20K, 30K, ptl00, ptl000, njl. ezichazwe ngumsebenzisi. I-K=10.0 okanye i-K=30 electrode isebenzisa indawo enkulu yesakhiwo seplatinum, emelana ne-asidi enamandla kunye ne-alkaline kwaye inomthamo onamandla wokulwa nongcoliseko; isetyenziselwa ikakhulu ukulinganisa ixabiso le-conductivity kwi-intanethi kwimizi-mveliso ekhethekileyo, efana nomzi-mveliso wokucoca amanzi amdaka kunye nomzi-mveliso wokucoca amanzi olwandle.
| I-electrode ehlala ihleli | 0.1 | 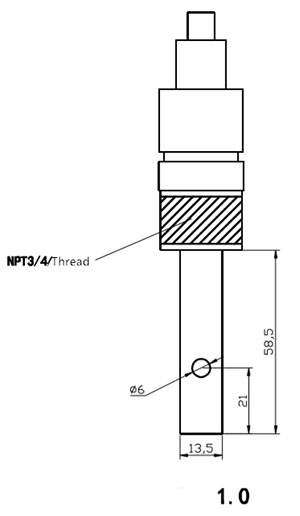 |
| Amandla oxinzelelo | 0.6MPa | |
| Uluhlu lokulinganisa | 0-200uS/cm | |
| Uqhagamshelo | Ufakelo lweMisonto eyi-1/2 okanye eyi-3/4 | |
| Izinto eziphathekayo | Intsimbi engagqwaliyo engama-316L | |
| Isicelo | Ishishini Lonyango Lwamanzi |
Ukuqhubayindlela yokulinganisa amandla amanzi okudlulisa ukuhamba kombane. Olu buchule lunxulumene ngokuthe ngqo noxinzelelo lwee-ion emanzini
1. Ezi ioni eziqhubayo zivela kwiityuwa ezinyibilikisiweyo kunye nezinto ezingaphiliyo ezifana ne-alkalis, ii-chloride, ii-sulfides kunye nee-carbonate compounds
2. Iikhompawundi ezinyibilika zibe zii-ion zikwaziwa ngokuba zii-electrolytes 40. Okukhona ii-ion zininzi, kokukhona ukuhanjiswa kwamanzi kuphezulu. Ngokufanayo, ii-ion ezimbalwa ezisemanzini, kokukhona ukuhanjiswa kwamanzi kuncinci. Amanzi anyibilikisiweyo okanye angena-ion angasebenza njengesithinteli ngenxa yexabiso lawo eliphantsi kakhulu (ukuba alikho kangako). Amanzi olwandle, kwelinye icala, anokuhanjiswa kwamanzi okuphezulu kakhulu.
Ii-ion ziqhuba umbane ngenxa yeentlawulo zazo ezilungileyo nezimbi
Xa ii-electrolytes zinyibilika emanzini, ziyahlukana zibe zii-particles ezitshajiswe kakuhle (cation) kunye nezitshajiswe kakubi (anion). Njengoko izinto ezinyibilikisiweyo zahlukana emanzini, amanqanaba e-charge nganye entle nembi ahlala elingana. Oku kuthetha ukuba nangona ukuhanjiswa kwamanzi kusanda ngee-ion ezongezelelweyo, ahlala engathathi cala ngombane 2






















